What is Ozone? How Does It Occur?
The CAS number of ozone is 10028-15-6. Ozone is a strong oxidant that can enter many chemical reactions with organic and inorganic compounds. Ozone’s oxidation potential is higher than many known chemicals. While the oxidation potential of the commonly used hydrogen peroxide is 1.77 eV, ozone comes after fluorine with 2.07 eV by 3.06 eV and hydroxyl radicals by 2.80 eV. Ozone, which has a very high oxidation potential, is also an effective sterile. Because of this feature, it has an important place in many sectors. Ozone turns into oxygen, its raw material, without leaving any residue.
Ozone is a nonlinear triatomic molecule. It has 2 oxygen bonds of equal length (1,278 ° A) and an average bond angle of 1160 49.
Ozone is a highly reactive gas, it can be irritating and toxic even at low concentrations. When ozone is inhaled for a long time, it produces symptoms such as irritation of the mucous membranes followed by headache. Although we do not have complete information about the problems caused by longer exposure to ozone, a decrease in lung capacity and lung disorders have been reported in animal experiments.
The employee health organization (OSHA -Occupational Safety and Health Administration) requires additional measures to be taken in case the ozone concentration exceeds 0.1 ppm. The 0.1 ppm limit is the limit for 8 hours of work per day for 5 days, in the Netherlands, the limit is 0.06 ppm, not 0.1. In cases of exposure not exceeding 15 minutes, the acceptable limit is 0.3 ppm. OSHA has set a limit of 0.2 ppm for exposures up to 2 hours. The discrimination threshold for the odor of ozone is about 0.02 ppm.
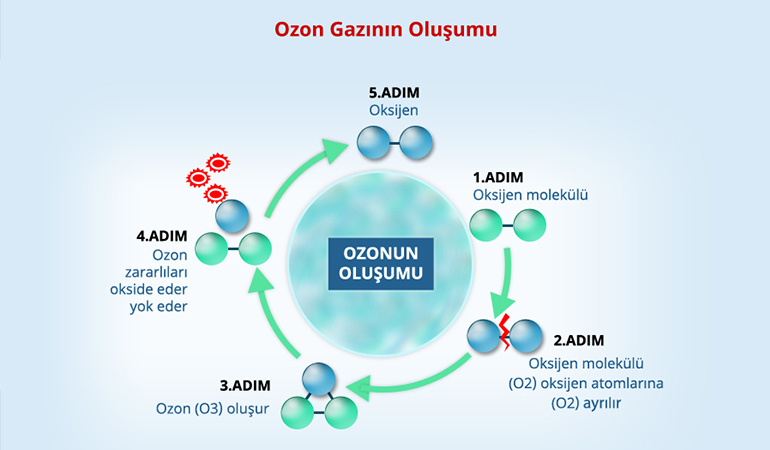
The basis for the formation of ozone is based on the formation of atomic oxygen radicals. These radicals then react with molecular oxygen to form ozone. Therefore, all the processes that convert molecular oxygen to oxygen radicals are potential ozone production reactions. The energies that make this possible are the electron or photon quantum energies. As this can happen in natural environments, electrons can be obtained from high-voltage Corona Discharge systems, chemonuclear sources and electrolytic processes, and the appropriate photon quantum energy can be obtained with ultraviolet rays below 200 nm and y-rays.
With the effect of the high-energy ultraviolet radiation from the sun, the oxygen molecule O2 in the atmosphere is broken down and the free oxygen turns into O2 atoms. Later, the free oxygen atoms O combine with the oxygen molecule with the effect of ultraviolet radiation to form the ozone molecule O3.
Ozone is also formed by the high-voltage electrical discharge that occurs during lightning. A fresh and clean fragrance is noticed after every lightning and downpour. This odor is the odor of ozone formed in the air. Industrially, the first of the two main methods for ozone production is the use of ultraviolet at 185 nm, the second is the dielectric method known as Corona Discharge, which has different applications in itself.
In the ozone production method with the use of ultraviolet, a lamp producing 185 nm UV light is used. During the passage of air around the UV lamp, oxygen molecules are split into oxygen atoms by UV effect. Since the formed oxygen atoms are not stable, they combine with oxygen molecules to form ozone.
The Corona Discharge CD method is an electrical discharge characterized by corona. It occurs by ionization of the fluid around the conductor when the electric field is strong enough. Conditions here should not allow short circuit or arc formation. It is the dielectric part that provides this. The electric charge is distributed over the dielectric surface, forming the corona. A plasma atmosphere is formed as a result of ionization and ions carry the charge to regions with lower potential. During corona formation, light, heat and sound are released.
The shape of the electrode, the quality of the gas and the discharge range are effective parameters. With the CD method, heat is released during ozone production and this heat must be removed from the generator. There are applications where the discharge tube is cooled with water or air to remove heat. When the two methods are compared, it can be said that the CD method is more advantageous than the UV method in terms of longevity, higher concentration of ozone in small sizes and operating costs. It is very important that the supply air is not humid in CD units.
Ozone can be described as a chemical compound formed by the combination of 3 oxygen atoms. It has an impressive scent. It is colorless.
It exists as oxygen atom, oxygen and ozone in the atmosphere. When the oxygen molecule, which consists of two oxygen atoms, specified as O2, is exposed to high voltage, it separates and combines with the oxygen molecule to form (O3) ozone, which is formed by 3 atoms. Ozone is an unstable gas. Ozone is formed by high electrical power caused by lightning in rainy weather and makes itself felt by emitting a fresh scent. Ozone gas surrounds the top of the atmosphere, filters the sun’s ultraviolet rays and sends them to the world. Ozone gas makes the sky appear blue.
The ozone layer is very important to life on earth. Substances containing chlorofluorocarbons damage the ozone layer, causing it to become thinner or perforate. These substances are chlorine derivatives, polystyrene foams, spray and airesols.
It is not possible to store ozone as it dissolves again at room temperature and turns into oxygen. It must be produced where and when it is used. Since ozone gas is an unstable molecule, its oxidation power is very high.







